✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 33903
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- 時間のかかるサブクローニングの手順は不要
- 昆虫または哺乳類細胞で翻訳後修飾を獲得
- 1つのコンストラクトが、3つの発現システムで効率的な発現を提供
Product Details
Performance
See figures
Principle
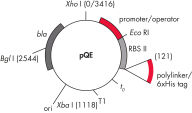
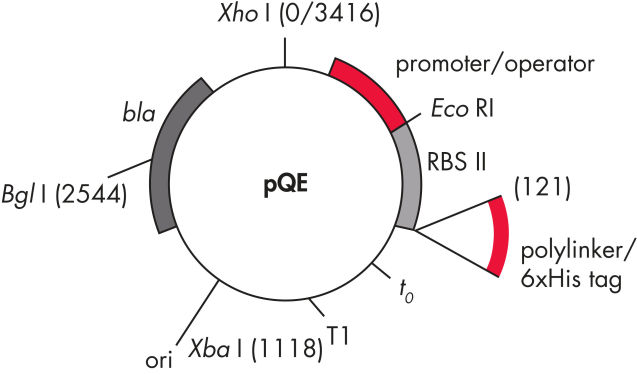
QIAexpress pQEベクターは、強力なファージT5プロモーター(E. coli RNAポリメラーゼによって認識)を二重のlacオペレーター抑制モジュールと組み合わせて、E. coliで遺伝子組み換えタンパク質の厳しく制御される、高レベルな発現を実現します。タンパク質合成は、高レベルのlacリプレッサーの存在で効果的にブロックされ、細胞毒性コンストラクトの安定性が向上します。pQEベクター(表と図、 pQE Vectorsを参照)は、遺伝子組み換えタンパク質のN末端またはC末端のいずれかの6xHisタグの配置を可能にします。
| 要素 | 説明 |
| 1. 最適化されたプロモーター/オペレーターエレメント |
ファージT5プロモーターおよび2つのlacオペレーター配列で構成されます。 これは、lacリプレッサー結合の確率を向上させ、確実にする 強力なT5プロモーターの効率的な抑制を保証します |
| 2. 合成リボソーム結合部位RBSII | 効率的な翻訳のために |
| 3. Hisタグをコードする配列 | ポリリンカークローニング領域への5’または3’のいずれか |
| 4. 翻訳終止コドン | 発現コンストラクトの便利な調製のため、すべてのリーディングフレーム内 |
| 5. 2つの強力な転写ターミネーター |
ファージλ由来のt0、およびE. coliのrrBオペロン由来のT1、防ぐために 読み過ごし転写を防ぎ、発現コンストラクトの安定性を保証するため |
|
6. ColE1複製開始点 |
pBR322由来 |
| 7. βラクタマーゼ遺伝子 (bla) | アンピシリン耐性を与えます |
See figures
Procedure
目的のタンパク質をコードするインサートは、適切なコンストラクト内にクローンされ、発現のために適切なE. coli株内に転換されます。発現は、IPTGの添加によって誘導されます。Vector pQE-TriSystemのコンストラクトは、E.coli内に転換されたり、昆虫細胞内での遺伝子組み換えタンパク質の発現のためにシャトルベクターとして使用されたり、哺乳類細胞内に導入されたりします。
Applications
QIAexpress Expression Systemシステムは、以下をはじめとする
数多くのアプリケーションに適したタンパク質の高レベル発現を実現します。
- 機能的にも構造的にも活性のあるタンパク質の精製
- 抗体産生のための変性条件下での精製
- 3次元構造決定のための結晶化
- タンパク質–タンパク質およびタンパク質–DNA相互作用を含むアッセイ
Supporting data and figures
pQE Vectors.
表に列記した番号の付いた要素。
Specifications
| Features | Specifications |
|---|---|
| In-frame cloning necessary | はい |
| Expression | In vivo |
| Tag removal sequence | いいえ |
| Expression species | 大腸菌、哺乳類 & 昆虫細胞 |
| Tag | 6xHisタグ |
| N- or C-terminal tag | C末端タグ |
| All three reading frames provided | いいえ |